
Berry to Underline its Global Healthcare Capabilities at CPHI Middle East
publié le mardi 03 décembre 2024
At this year’s CPHI Middle East, packaging specialist Berry Global will underline its extensive capabilities in pharmaceutical packaging, medical devices and drug delivery solutions, demonstrating the company’s commitment to supporting the needs of healthcare providers and patients throughout the Middle East and other emerging markets.
Berry will showcase a variety of standard and tailored products in areas such as child-resistant and senior-friendly packaging, ophthalmic, nasal, and container platforms, respiratory drug delivery devices, and medical packaging. All provide customers with consistent quality and reliability of supply to meet their individual requirements.
With growing demand for high-quality, certified child-resistant packaging solutions in the pharmaceutical sector, Berry will highlight the PharmaSafe® child-resistant closure, which meets the latest ISO 8317 standard: Child-resistant packaging – requirements and testing procedures for re-closable packaging. The company has recently increased production capacity for the closure, which can now be paired with Berry’s brown PET bottles in 100, 150, 200 and 250ml sizes.
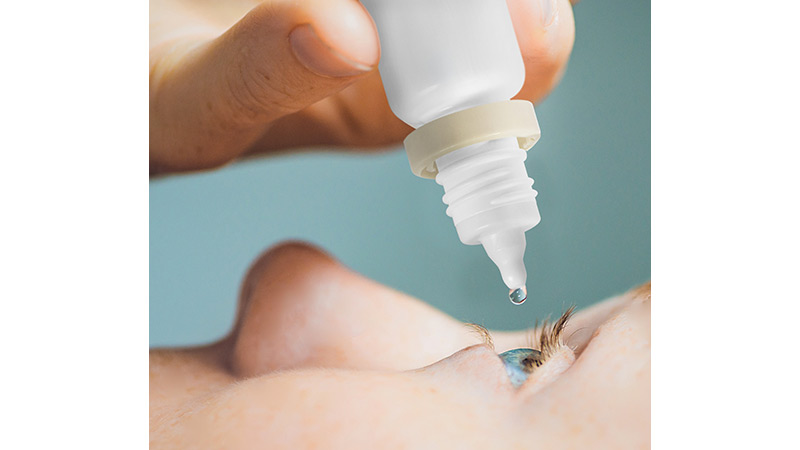
From its ophthalmic ranges, Berry will introduce its recently launched easy-squeeze ophthalmic 10ml bottle that has been qualified for use with Aptar Pharma’s globally recognised Ophthalmic Squeeze Dispenser (OSD). The bottle provides a reliable and user-friendly complement to the OSD, which is a leading solution for preservative-free prescription and over-the-counter products, featuring proven microbiological integrity and supporting preservative-free solutions to treat a wide range of eye conditions such as Conjunctivitis and Glaucoma.
Berry will also demonstrate its full range of three-piece eye droppers, Rispharm™ R2. The enhanced design for the Rispharm™ R2 multidose eye dropper provides an improved patient experience in terms of safety and convenience, while maintaining its excellent drug protection and delivery performance. In particular, the eye dropper is in line with the FDA recommendation to ensure that the tamper evident ring for any eye dropper stays connected to the bottle after opening with no risk of it falling off into the patient’s eye during administration of the product. The company will also present its extensive range of pill jars, which are available with a variety of neck finishes to meet the needs of a wide variety of products and applications.











