Innovative solution to decontaminate N95 mask
posted Friday 24 April 2020
As a global manufacturer of drug delivery, consumer product dispensing, and active packaging solutions, AptarGroup is seeking U.S FDA Emergency Use Authorization (EUA) for a solution that allows easy disenfecting of N95 filtering facepiece respirators (N95 mask), which are desperately needed by healthcare personnel due to the Covid-19 pandemic.
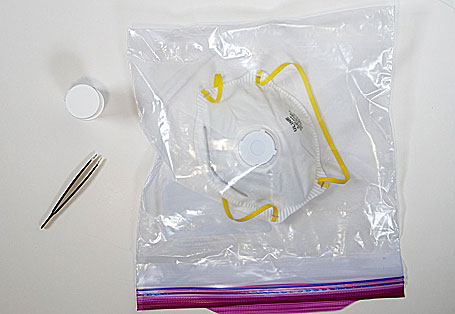
In this simple process, the N95 mask and a small strip of Aptar’s ActivShield are placed inside any commonly available one-gallon plastic bag. The strip releases a controlled amount of chlorine dioxide inside the sealed bag to decontaminate the mask. The process takes about three hours until the mask is ready to wear again. It can be performed on-site at the local hospital where the mask is being used. The company is working to provide approximately four million ActivShield strips per week and is working to expand its production capacity with the intent to deliver ten million per week by the end of April.
All rights reserved except agreement written by Emballage Digest or mention of the magazine










