TekniPlex Healthcare unveils solutions for single-use bioprocessing and cell & gene therapies
posted Wednesday 17 April 2024
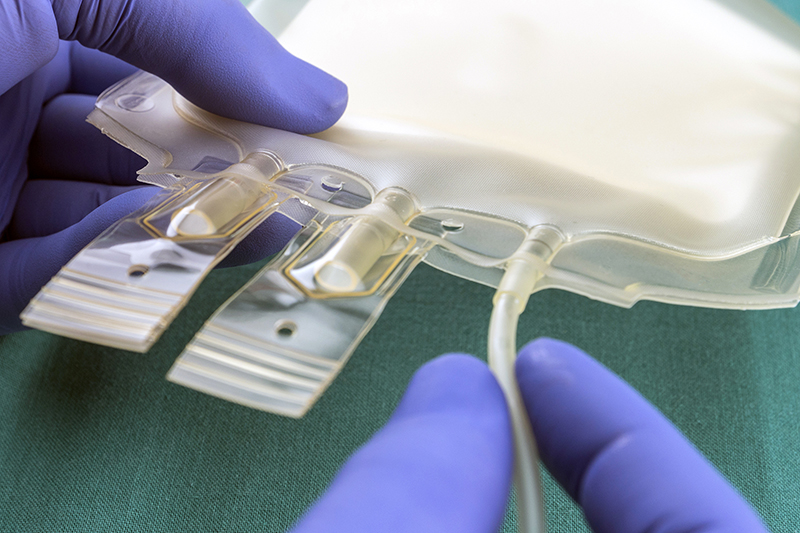
TekniPlex Healthcare is currently emphasizing its expertise and intellectual property related to the single-use bioprocessing and the cell and gene therapy markets at Interphex NYC. The company highlights its portfolio for the burgeoning biologics niche, for which it supplies complementary solutions across the upstream, downstream processing and final fill operations.
Solutions include an array of customizable barrier cleanroom films, tubing such as weldable TPE and TPV, silicone and PVC, as well as various compounds for associated molded components.
For cell and gene therapy applications, TekniPlex Healthcare is a leader in the production and supply of tubing in a variety of configurations, grades and durometers. Both single- and multi-lumen constructions are available, as are multi-layer versions incorporating complementary materials for performance optimization.
For traditional bioprocess applications, TekniPlex Healthcare produces tight-tolerance biopharm-grade constructions comprised of TPE, TPV and silicones. For each of its offerings, the company’s manufacturing prowess boasts extrusion across four continents, and covers all stages of the drug development process, from small batch production through scale-up and commercial quantities.
Also at Interphex NYC, TekniPlex Healthcare demonstrates its coextrusion film capabilities, including newly expanded cleanroom manufacturing capacity for multilayer films suitable for single-use bioprocessing applications. Situated in the company’s Puurs, Belgium facility, the new ISO 7 cleanroom multilayer blown film line joins existing cast coextrusion ISO 7 cleanroom manufacturing capacity suitable for bioprocessing bag film production. The additional film production further expands the company’s capabilities to provide customizable film solutions to bioprocessing bag manufacturers, with a wide range of potential resin combinations helping meet durability needs, as well as extractables and leachables profile requirements.
The company’s assets also include a pilot film coextrusion line in its Global Innovation Center in Holland, OH for quick prototyping, helping biopharma-focused companies iterate film options quickly to reduce go to market time and costs. The company also has recently installed cleanroom bag prototyping equipment allowing it to assemble 2-D single-use bioprocessing bags for concept validation and application testing purposes.










